Living with your new Joint Replacement
Getting a Good Night’s Sleep after Hip or Knee Replacement Surgery
One of the most common complaints after total joint replacement is difficulty sleeping. The most common cause of sleep disruption is pain. It has been reported that more than half of patients wake up with pain after joint replacement. Many factors can affect the quality of sleep after a major surgery including anesthesia-type, narcotic use and discomfort due to pain or restricted leg movements.
As sleep is crucial to the recovery process, it is important to follow appropriate pain management protocols.
Contemporary pain management protocols are designed to be multifaceted and inhibit pain in a multitude of ways. Many protocols use a variety of injections and nerve blocks for localized pain, as well as employing narcotics and anti-inflammatory medication for several weeks after surgery. As such, pain protocols should be fully followed to ensure an adequate recovery.
Usually around the second or third week after surgery, you will start to increase your activity levels while at the same time decrease your narcotic use. This often coincides with having a difficult time sleeping. When this occurs, you should take your pain medication an hour before bed to achieve better comfort and help restore your sleep cycle. A few days off from strenuous activity or physical therapy will not inhibit your recovery, but can have a tremendous effect on your ability to fall asleep and stay asleep.
Overall, sleep deprivation after total joint replacement is manageable through pain management, the occasional use of sleeping pills, and activity modification. If all else fails, it is advisable to call your surgeonwho can help you manage sleep disturbances during the postoperative period.
Source
- Rosenberg-Adamsen S, Kehlet H, Dodds C, Rosenberg J. Postoperative sleep disturbance: mechanisms and clinical implications. Br J Anaesth.1996;76:552-559.
- Wylde V, Rooker J, Halliday L, et al. Acute postoperative pain at rest after hip and knee arthroplasty: severity, sensory qualities and impact on sleep. Orthopaedics & Traumatology: Surgery & Research. 2011;7:139-44.
- Myoji Y, Fujita K, Mawatari M et al. Changes in sleep-wake rhythms, subjective sleep quality and pain among patients undergoing total hip arthroplasty. Int J Nurs Pract. 2014 Apr 30. doi: 10.1111/ijn.12345. [Epub ahead of print]
- Krenk L, Jennum P, Kehlet H. Sleep disturbances after fast-track hip and knee arthroplasty. Brit Journ of Anesthesia. 2012; 109:769-75.
Will my artificial joint set off airport security metal detectors?
Belt buckles, key chains and smartphones may set off sensitive metal detectors at airport security checkpoints. Many commonly used orthopaedic implants may also set off the metal detectors. Over 90% of implanted total hip and knee arthroplasty devices will set off airport metal detectors. Many implants now include ceramic and plastic materials in addition to metal, and the metal will still likely cause an alarm.
A card from your physician is no longer needed for identification of these type of implants.
If you or a family member has a metal implant, he or she should inform a Transportation Security Administration (TSA) officer before screening begins. Passengers can use the TSA’s Notification Card to communicate discreetly with security officers; however, showing this card or other medical documentation will not exempt a passenger from additional screening.
Many patients now prefer to be screened by imaging technology (X-ray machine) to reduce the likelihood of a pat-down being necessary. If a pat-down is selected by the TSA, it will be helpful to wear clothes that allow you to easily reveal your surgical scar.
The TSA offers more information on metal implants TSA Notification Card on their website.
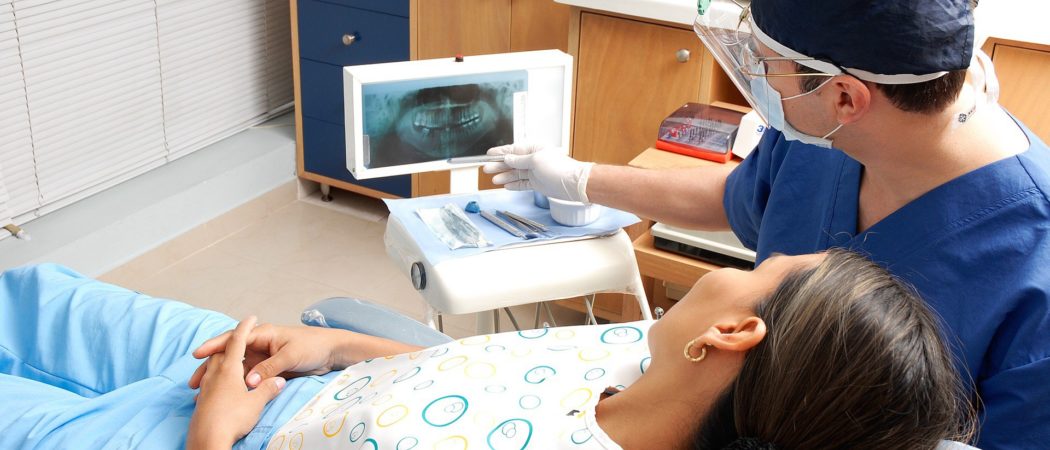
Preventing Infection in Your Joint at the Dentist’s Office
During a dental procedure, it is possible for bacteria from the mouth, teeth or gums to travel through the bloodstream and settle in an artificial joint. The use of an antibiotic pill prior to dental work has been thought to lower this risk. Orthopedic surgeons have historically recommended the routine use of antibiotics prior to dental work due to the catastrophic effects of a prosthetic joint infection and the relative safety of a single dose of oral antibiotics.
Guidelines
In 2013, The American Academy of Orthopaedic Surgeons and The American Dental Association worked together to create guidelines for this situation. The workgroup reviewed the available published data to try and synthesize recommendations for patients and practitioners. Unfortunately, there is not a large amount of quality data, but they issued three findings:
- The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.
- We are unable to recommend for or against the use of topical oral antimicrobials in patients with prosthetic joint implants or other orthopaedic implants undergoing dental procedures.
- In the absence of reliable evidence linking poor oral health to prosthetic joint infection, it is the opinion of the work group that patients with prosthetic joint implants or other orthopaedic implants maintain appropriate oral hygiene.
Factors to Consider
Many factors should be considered when you are making this decision, such as:
- The type of procedure being performed – routine cleaning vs. more invasive work
- Your health status – patients with compromised immune systems are at greater risk
- The presence or absence of an active infection in the mouth
- The recommendations of your surgeon and dentist
With the lack of a definitive answer on this question, we recommend that you discuss this with your surgeon.
Antibiotics to Use
If your surgeon or dentist recommends antibiotics, the following antibiotics are usually used:
- If you are NOT allergic to Penicillin, 2 grams of Amoxicillin, Cephalexin, or Cephradine taken one hour prior to the procedure.
- If you ARE allergic to Penicillin, 600mg of Clindamycin taken orally or administered by injection one hour prior to the procedure.
Developing an infection in and around a total hip or knee replacement is one of the most catastrophic complications that can occur. If you suspect you might have an infection, it is important to seek treatment early.
Addressing Implant Recall Concerns
How reliable will my new joint be?
Recalls of hip and knee replacement implants can cause understandable concern on the part of both patients and physicians. Those who have had joint replacement surgery with implants that were subsequently recalled may wonder if their health will be compromised or if they will need further surgery. If you are considering joint replacement surgery, you may be apprehensive about the longevity of the implants utilized.
Orthopaedic surgeons, national databases of implant performance called “registries,” as well as implant manufacturers closely scrutinize outcomes of joint replacement implants. Fortunately, implant recalls are rare and affect a very small fraction of the more than seven million patients with hip and knee replacements. Recall of a specific implant does not mean that all patients with that implant will have adverse health consequences, or require surgery to have the implants removed. Many implants have been utilized in joint replacement surgeries for over ten years without ever being recalled, and these implants will likely never be recalled.
If an implant is recalled, it cannot ever be used in a joint replacement surgery again.
Despite extensive laboratory testing, implants with newer technologies aimed at improving patient outcomes may have unacceptably high failure rates once used in large numbers of patients. If such an implant is recalled, the manufacturer notifies surgeons who have used the implant.
Your joint replacement surgeon can most effectively discuss implications of an implant recall, and serve as your advocate, if you are affected. Surgeons will closely monitor patients with recalled implants to ensure that they remain healthy and intervention is prompt if a problem is found. Contact your joint replacement surgeon with any questions you have about implant recalls.
Source
This article has been written and peer reviewed by the AAHKS Patient and Public Relations Committee and the AAHKS Evidence Based Medicine Committee. Links to these pages or content used from the articles must be given proper citation to the American Association of Hip and Knee Surgeons.